Instructions for Authors
Authors’ Guideline
All manuscripts must be submitted via the online system. Manuscripts submitted for publication must be prepared according to the guideline given below.
Template in MS Word: Microsoft Word template
References Style file for EndNote: Vancouver
This guideline is intended to assist authors as they prepare their manuscripts. To avoid any delay and time-consuming restructuring, Plant Science Review (PSR) asks and encourages authors to read the guidelines before writing the manuscript.
Plant Science Review (PSR) publishes review and research articles. All papers must be written in English, and follow a clear, concise style. The language editors may have to check the language and grammar of your submitted manuscript, and make editorial changes if deemed necessary.
Article Types
Research Articles
A Research article is a detailed technical report of an original study that is likely to impact its field. It is a primary report where authors collect and analyze data and draw conclusions from the results leading to an original study in the literature. Research articles incorporate a comprehensive list of elements. There is no specific word count limitation; however, manuscripts must be as concise as possible.
Review Articles
A Review article is a paper based on other published research. It is a secondary source. It does not report original research but rather critically evaluate previously published material. Typically, a review article analyzes or synthesizes existing literature on a subject with the aim of expanding on its current understanding or sums up the already existing work to relate it to its present status and suggest new research directions. Structured reviews and meta-analyses should use the same structure as research articles and adhere to the PRISMA guidelines, and authors should also include a completed PRISMA checklist and PRISMA flow diagram as supporting files.
Guidelines for Cover Story
We select cover images for their scientific interest and aesthetic appeal. The editorial team will select those images they consider most appropriate for the cover positions and contact the chosen images’ authors. The cover images should be in an original, high-resolution format, and editable vector graphic images are preferred. Authors may receive request emails for permission to use their images as a cover story by us. Please send your approval and along with the electronic files, including a short legend concisely explaining the image (50-60 words).
The cover of the journal is editorial material. As such, the editors reserve the right to adapt it during the final design process or ask you to make changes to your suggestion. No charge is required at present for Cover Story.
Other Requirements:
- Image type: TIFF/PSD files are preferred
- Design suggestion: A cover is a showcase for your research and the journal. It should be eye-catching and make people want to learn more about your article.
- Image layout: The image should have a full-page layout. Space should be available for the journal-title and other information on the page, but aside from that, the full area is available for your image.
- Copyright: Any cover must be a completely new design. It is not appropriate to use elements taken from third-party content, such as in previously published work or any elements that are otherwise subject to copyright or licensing restrictions, as the license should be transferable to Plant Science Review (PSR) -.
Cover Letter
A submitted manuscript must be accompanied by a cover letter. The cover letter must clearly state that the manuscript is an original work with its own merit, has not been previously published in whole or in part, and is not being considered for publication elsewhere. It should also include statements clearly indicating that all authors have read the final manuscript, have approved the submission to the journal, and have accepted full responsibilities pertaining to the manuscript’s delivery and contents. If there are any ethical, copyright, disclosure issues that come with the manuscript, please reveal them in the cover letter. In the cover letter, authors need to declare that there is no conflict of interests or disclose all the conflicts of interest regarding the manuscript submitted.
Templates
Use the Microsoft Word template or LaTeX template to prepare your manuscript.
Authors are strongly encouraged to use either the Microsoft Word or LaTeX template to prepare their manuscript. Using the provided template will significantly speed up the copy-editing and publication process for accepted manuscripts. The total file size for all submissions must not exceed 200 MB. If the file size exceeds this limit, please contact the Editorial Office at editor@crcjournals.org. Accepted file formats are:
- Microsoft Word: Manuscripts prepared in Microsoft Word must be submitted as a single file. When using Microsoft Word, we recommend using the Microsoft Word template (each section has a predefined style, accessible via the “Styles” gallery in Word). Figures should be inserted into the main text immediately after the paragraph where they are first cited.
- LaTeX: Manuscripts prepared in LaTeX should be submitted in a single ZIP folder containing all source files and images, allowing the Editorial Office to recompile the PDF. We recommend using the LaTeX template for LaTeX manuscripts.
- Supplementary Files: These can be in any format, though it is recommended to use common, non-proprietary formats whenever possible.
General Format of Articles
Manuscripts should comprise:
Front matter: Title, Author list, Affiliations, Abstract, Keywords.
Main text: For Articles, a structured format, e.g., Introduction, Results, Discussion, Methods, Conclusions, is recommended, while Reviews may use a more flexible structure.
Back matter: Acknowledgment, Funding Statement, Author Contributions, Availability of Data and Materials, Ethics Approval, Conflict of Interests, Supplementary Materials (if any), Glossary (if any), Appendices (if any), and References.
Front Matter
● Title
The title of your manuscript should be precise, clear, and directly related to the study. It should indicate whether the research involves human or animal trial data, or if it is a systematic review, meta-analysis, or replication study. Avoid including short forms like running titles or headers, as these will be removed by the Editorial Office. Acronyms are not allowed in paper titles. They can be used in abstracts only if the related expanded form is provided (just after the acronym, in parentheses) the first time they are used.
● Author Information
Please provide the full names of all authors, including first and last names, with middle name initials included if necessary. Affiliations should follow the PubMed/MEDLINE standard format, including detailed address information such as city, postal code, state/province, and country. At least one author must be designated as the corresponding author, whose email address will be publicly displayed in the published article. Please note that after acceptance, changes to author names or affiliations may not be permitted. For authors with equal contributions, use a superscript symbol (#) for identification and include the following statement below the affiliation: “These authors contributed equally to this work.” Additionally, equal contributions should be clearly detailed in the author contributions section. Please review the relevant criteria to ensure authorship qualifications are met.
● Author Affiliations
All authors must include their current affiliation as well as the institution where the majority of the research for the manuscript was conducted. The primary affiliation should typically reflect the institution that provided the most support or where the bulk of the research was performed, but authors are encouraged to confirm any specific requirements with their institution, particularly regarding contractual agreements.
Accurate author names and affiliations are crucial to ensure proper attribution, citation, and to avoid issues related to recognition, promotions, or funding. Once an article is published, requests for updates or corrections to author details, including affiliations, may not be accommodated.
For Independent Researchers Authors who are not currently associated with any university, institution, or organization, and were not during the development of the manuscript, should identify themselves as “Independent Researchers.”
● Abstract
Abstracts of a research paper should be typically 200 to 400 words in length, and 150 to 300 words for a review paper. Abstracts shall be running continuously and shall not include reference citations. Abbreviations that appear only once in the abstract should be defined in full. If abbreviations appear more than once, the full definitions should be provided first before they can be used elsewhere.
● Keywords
Please list 3 to 10 pertinent keywords specific to the article yet reasonably common within the subject discipline.
Main Text
● Figures and Tables
Order
- Figures and tables should be numbered consecutively using Arabic numerals and placed within the text immediately following their first citation to maintain a seamless flow and clarity in the presentation, and the first citation of figures and tables in the main text must follow a sequential order (as well as text Boxes and Tables). In the published article, the figures are inserted based on the placement of the first citation and caption.
- The lettered subpanels of whole figures may be cited in any order in the text following the first mention of each whole figure in numerical order. For example, any subpart of Fig. 3 may be cited in any order (e.g., Fig. 3C before Fig. 3A) provided that Figs. 1 and 2 have already been cited.
Content
- The figure content should be complete and the characters should not be masked. Unnecessary marks such as red wavy lines and hard (soft) returns are not allowed in figures;
- Any special characters or icons in an image/table (e.g., *, **, ***, #, …) need to have a corresponding explanation (can be added in the image or caption);
- Please remove all non-English terms or add a definition for them;
- References in the form of “[xx]” are not allowed in images. “Author + Year” format can be used in the image, and all mentioned references must be cited in the caption if necessary;
- No specific feature of an image should be augmented, altered, enhanced, obscured, moved, or removed. The focus should be on the data rather than its presentation (e.g., background, imperfections, and non-specific bands should not be “cleaned up”);
Resolution and Format
- Figures should be scaled to a maximum width of 16.51 cm (6.50 in) and height of 20 cm (7.87 in), preserving their original proportions without distortion. Any excess white space surrounding a figure should be removed before calculating its size. The preferred format is .tif, with RGB color space, a DPI of 500+ (accepted image resolutions: Line Art ≥ 900 dpi, Halftone ≥ 300 dpi, Combo ≥ 600 dpi), no alpha channels, and flattened layers;
| Image Type | Description | Recommended Format | Resolution |
| Line Art | An image composed of lines and text, which does not contain tonal or shaded areas | tif or eps | 900–1200 dpi |
| Halftone | A continuous tone photograph, which contains no text | tif | 300 dpi |
| Combo | Image contains halftone + text or line art elements | tif or eps | 500–900 dpi |
2. To avoid any errors during position changes, please provide the combined image instead of editable pieces in the figure;
3. Please provide an editable table in MS Word format, not images (including algorithms, listings, etc.);
4. RGB (8 bit/channel), CMYK, or greyscale mode are acceptable;
5. Do not use Photoshop or such software to change the color or appearance of figures.
Labels and Captions
- Figure labels must be sized in proportion to the image, sharp, and legible. The label size should be no smaller than 8-point and no larger than the font size of the main text;
- Labels must be saved using standard fonts (Arial, Helvetica or Symbol font) and should be consistent for all the figures;
- All labels should be in black, and should not be overlapped, faded, broken or distorted, feature unnecessary gaps or irregular spacing, or appear condensed, expanded, or otherwise distorted either horizontally or vertically;
- Space must be inserted before measurement units. The first letter of each phrase, not each word, must be capitalized.
- Provide a short title (in the legend, not on the figure itself) and an explanation in brief but sufficient detail to make the figure intelligible without reference to the text. Statistical evaluations should indicate the test used.
- Each panel of a multi-panel figure (referred to as, e.g., Figs. 1A, 1B, 1C, and 1D in the text) should be logically connected to the other panels, and all of the panels should be assembled into a single file on a single page. Images that contain large amounts of information should be broken down into multiple figures to ensure that all of the information is visible. To repeat, multiple panels must be assembled and submitted as a single file rather than as separate files.
- The sublabels for panels (again, referred to as, e.g., Fig. 1A, 1B, 1C, and 1D in the text) should be placed in the top left-hand corner of the panels and contrast clearly with the background. Note that each panel should be labeled with only a letter (e.g., A, B, C, and D—not 1A, 1B, 1C, and 1D).
Scale Bar
- A scale bar, rather than magnification, must be provided for any micrographs. The scale bar should be explicitly and prominently displayed on each figure; one cannot simply add a line on the figures without any scale bar description or simply add a description of the scale in the figure legend without a scale bar in the figure. This practice is mandatory for all micrographs and petri dish diagrams.
Copyright of Figures and Tables
- Ensure that permission has been obtained and there is no copyright issue. If copyright is needed, please provide a citation in the following format: “Reprinted/adapted with permission from reference [xx]. Copyright year, copyright owner’s name”. All figures, tables, and images will be published under a Creative Commons CC-BY license, and permission must be obtained for the use of copyrighted material from other sources.
- If a figure or table has been published previously (even by an author of the manuscript being submitted for review), copyright permission for reuse of the figure or table will often be required. The acknowledgment and written permission from the copyright holder will be required where necessary. It is the responsibility of the authors to acquire the licenses, follow any citation instructions requested by third-party rights holders, and cover any supplementary charges.
- For any figures (or tables) that contain data from a public database (e.g., Gene Ontology/KEGG), the source should be cited in the caption, legend, or title explicitly. For publicly available DNA sequences, the accession number should be provided.
Images of Gels and Blots
- Images of gels and blots in figures should not be over cropped around the bands of interest. Rather, figure panels should include some background area above and below bands. Any non-specific bands from the original image should be included in the figure and explained in the text or figure caption or legend..
- When a comparative analysis of bands is presented, all of the relevant samples should be run on the same gel/blot.
- Each figure should include all of the relevant controls, and, when appropriate, control samples should be run on the same blot or gel alongside the experimental samples.
- A figure panel should not include composite images of bands originating from multiple blots, exposures, or gels. If data from multiple blot or gel images are necessary to illustrate the results, the various images should be clearly distinguished as separate panels within the figure (not spliced together), and the caption or legend should make clear that multiple gels, blots, or exposures are being presented.
- Any rearrangement of lanes from a single blot/gel image during the preparation of a figure as well as any image splicing should be clearly indicated with vertical black lines on the figure, and the caption or legend should explain how the figure was made. The addition of the lines would be appropriate, for example, when fragments of the same original image have been spliced together to re-order lanes or to remove irrelevant lanes.
- Quantitative comparison of samples across multiple gels/blots is strongly discouraged. If such comparison is unavoidable, the figure legend must state whether the samples derive from the same experiment or parallel experiments and whether the gels/blots were processed in parallel.
- The rearrangement of lanes that are non-adjacent in a gel must be clearly indicated in a manner that delineates the boundary between the lanes and should be acknowledged in the figure caption or legend.
- Loading controls (e.g., GAPDH, actin) must be run on the same blot. When sample processing controls are run on different gels, this fact must be acknowledged in the caption or legend. Any cropped images of gels must retain all of the important bands.
- High-contrast gels and blots are discouraged because overexposure may mask additional bands.
- Authors should take care to (1) check figures for duplications, (2) check blots and gels for the splicing of lanes, (3) indicate whether panels show sample processing or loading controls, and (4) ensure that the unprocessed scans provided match the figures.
Figure Samples
From: Song Q, Zhang J, Zhang Q, Liu J, Lv K, et al. Exosomes derived from circBCRC-3-knockdown mesenchymal stem cells promoted macrophage polarization. BIOCELL. 2020;44(4):623–29.
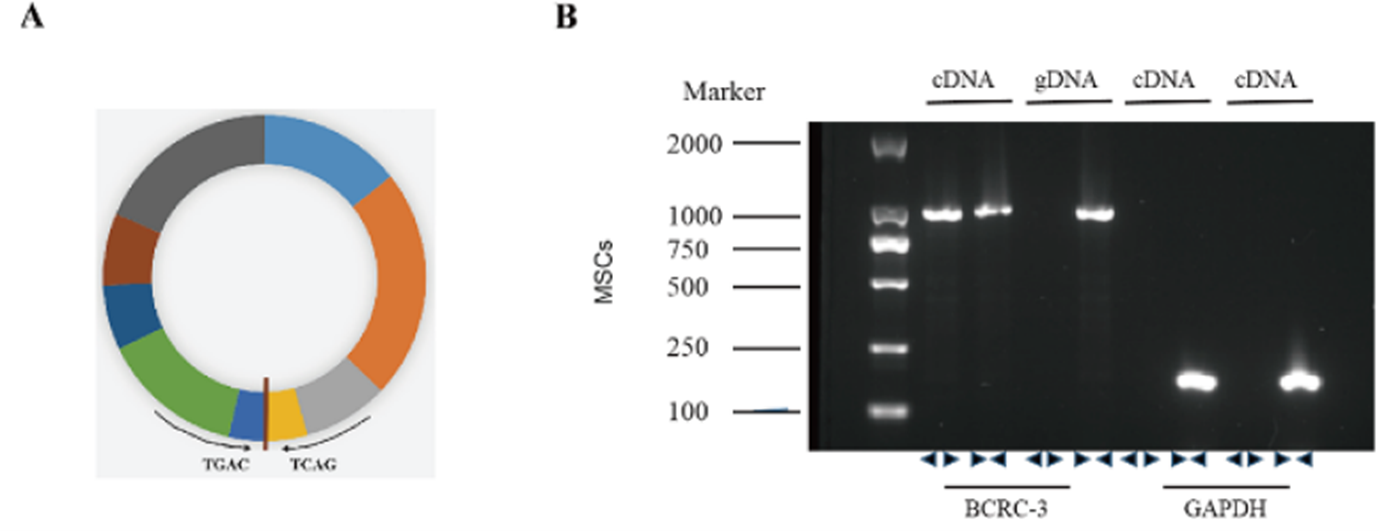
Figure 1: Identification of circBCRC-3 in MSCs. (A). The genomic structure indicates that circular RNA BCRC-3 consists of nine exons (1002 bp) from the PSMD1 gene. (B). Agarose gel electrophoresis analysis of PCR product with divergent and convergent primers of circBCRC-3 in cDNA and gDNA. GAPDH was used as the negative control
From: Zhang J, Huang Y, Liu W, Li L, Chen L. Chaperone-mediated autophagy targeting chimeras (CMATAC) for the degradation of ERα in breast cancer. BIOCELL. 2020;44(4):591–95.
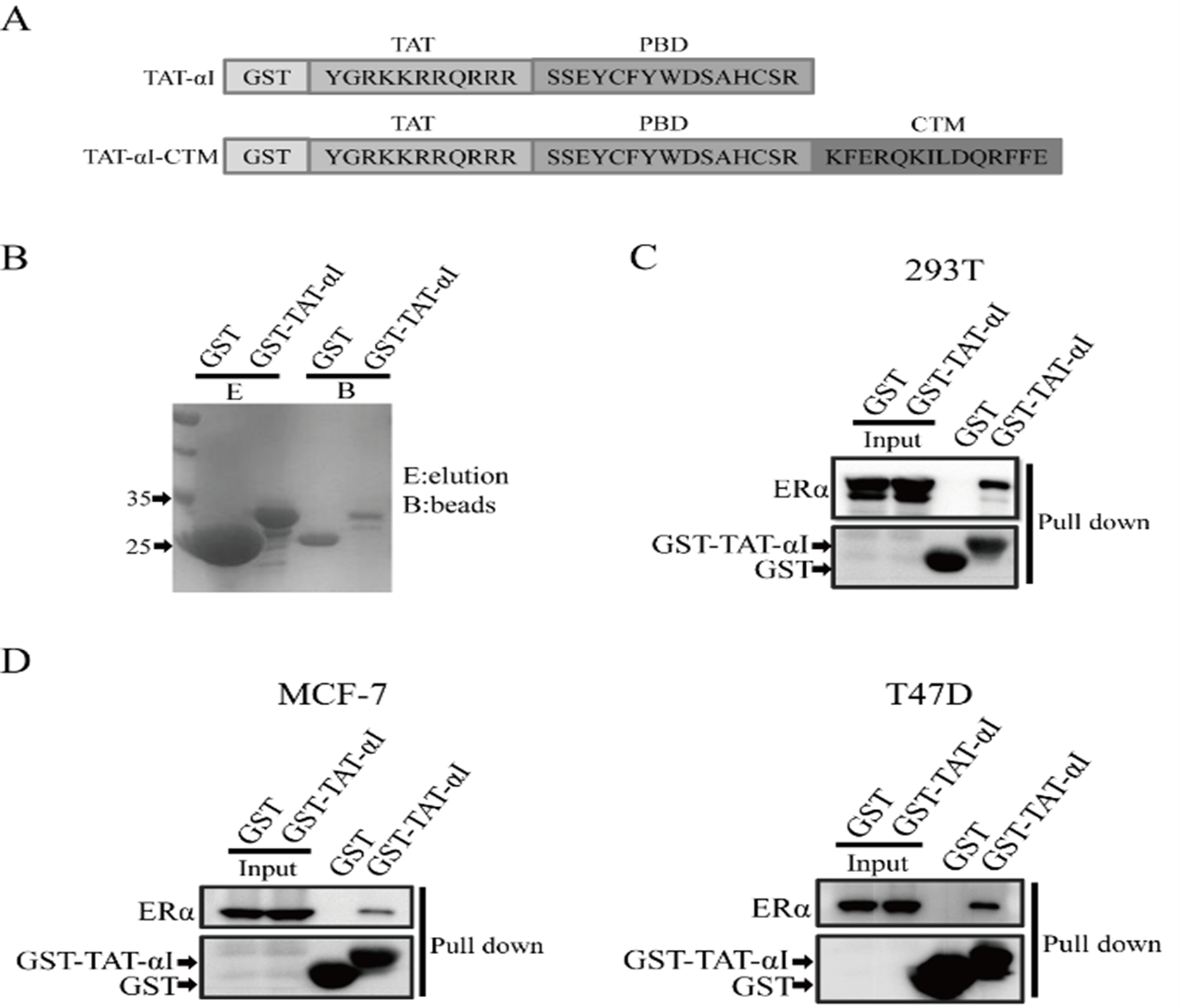
Figure 2: The binding of peptide αI to ERα. (A) Design TAT-αI-CTM and TAT-αI peptides. (B) Production of GST and GST-TAT-αI using an E. coli expression system. Coomassie blue staining after SDS-PAGE assessed their purity. (C) Pull-down of TAT-αI and ERα. HEK 293T cells were transiently transfected with plasmids pEGFP-N2-ERα. 48 h after transfection, cell lysates were subjected to GST pull down, and the pull-down fractions were immunoblotted analyzed. (D) Pull-down of TAT-αI and ERα in ERα-positive breast cancer cell lines, MCF-7 and T47D
From: Fu S, Yun T, Ma D, Zheng B, Jiang D, et al. Thylakoid Transit Peptide Is Related to the Expression and Localization of NdhB Subunits in Soybean. Plant Science Review (PSR) -Int J. Exp Bot. 2021;90(1):99–110.
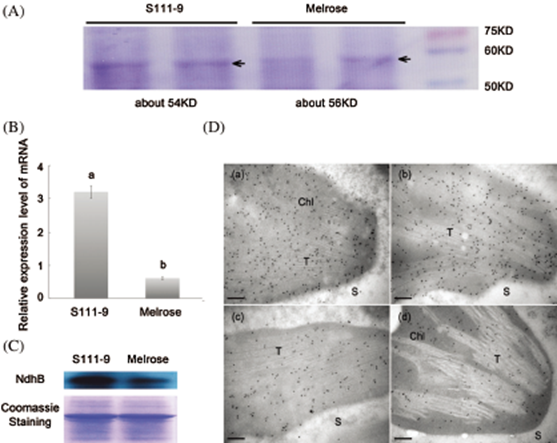
Figure 3: Comparisons of sizes, relative abundance of mRNA and protein, and immunolocalization for NdhB subunits in Melrose and S111-9. (A) Sizes of NdhB subunits were analyzed by SDS-PAGE. NdhB proteins were isolated and purified from the leaves of Melrose and S111-9 extracted with Crosslink IP Kit. (B) Relative mRNA expression level of ndhB in Melrose and S111-9. Bars represent the mean ± SD of three biological replicates. Student t-test was applied to assess difference of means between two varieties. Different letters indicate a significant difference between varieties (p < 0.05). (C) The content of NdhB were analyzed using western blotting in the leaves of Melrose and S111-9. The equal loading of lower pictures is shown by coomassie staining. (D) Immunolocalization of NdhB subunits in S111-9 (a,b) and Melrose (c,d). All immunogold particles were mainly concentrated in chloroplast, not in the cytosol. T, Thylakoid; S, Cytosol Stroma. Scale bars = 0.2 μm
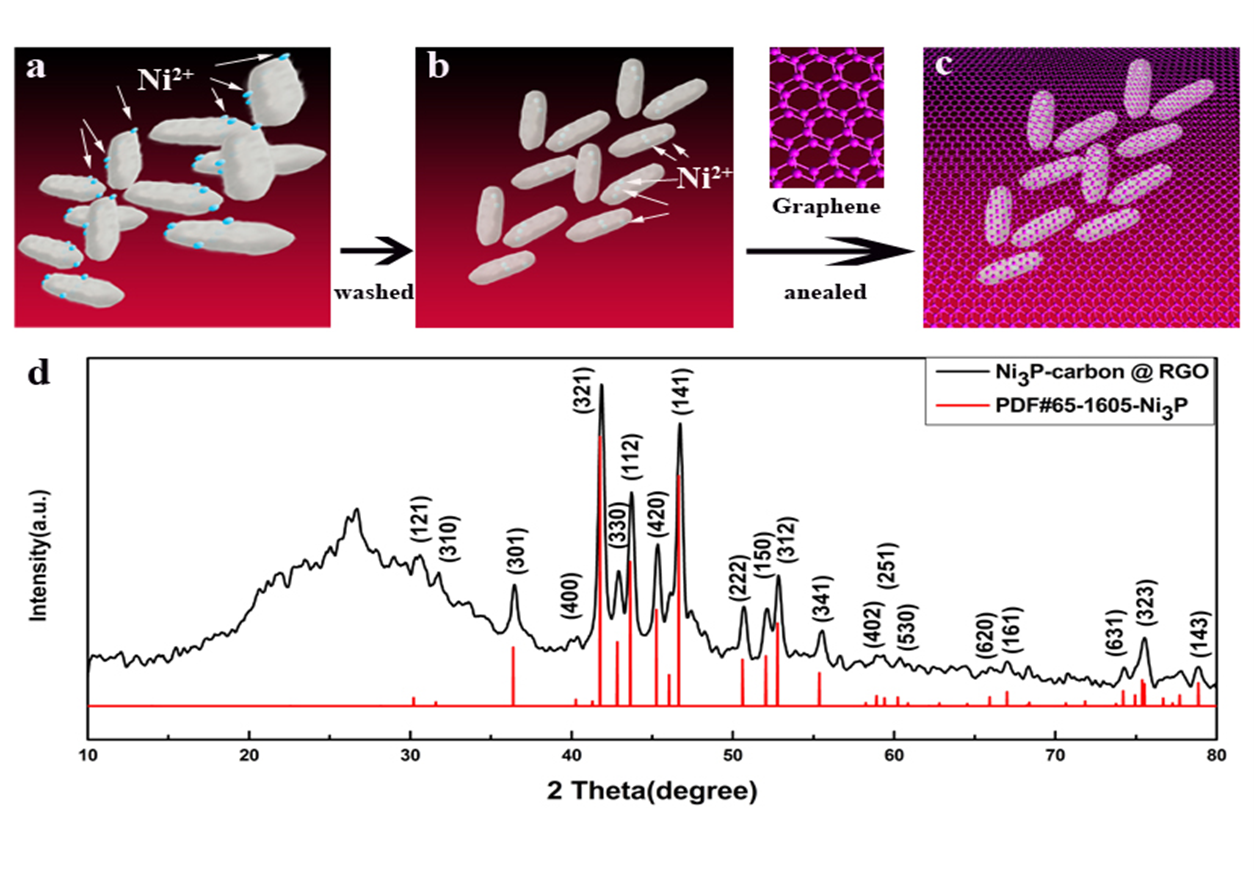
From: Yang Y, Xu K, Zhao B, Liu N, Zhou J. The Bacteria Absorption-based Yolk-Shell Ni3P-Carbon @ Reduced Graphene Oxides for Lithium-Ion Batteries. J Renew Mater. 2021;9(5):855–65. Doi:10.32604/jrm.2021.014525.
●Acronyms/Abbreviations/Initialisms
Acronyms, abbreviations, and initialisms must be defined upon their first use in three sections: the abstract, the main text, and the first figure or table. When first introduced, include the abbreviation or acronym in parentheses following the full term. Accepted abbreviations for statistical parameters are: P, n, SD, SEM, df, ns, ANOVA, t.
● International System of Units
The use of SI Units (International System of Units) is required. Whenever possible, convert imperial, US customary, or other units to SI equivalents.
- There should be a space between the unit and Arabic number: 5 mm NOT 5mm.
- There should be a space before and after the operator: 3 cm × 5 cm NOT 3 cm×5 cm.
- Please use Arabic number and SI Units (International System of Units) in the manuscript: 5 kg NOT five kilograms or 5 kilograms or five kg.
- Do not use hyphen/dash or any connector symbol between the value and its unit: 5 kg NOT 5-kg
- Please clarify all units during a calculation or a mathematical relationship: 3 cm × 5 cm NOT 3 × 5 cm, 123 g ± 2 g or (123 ± 2) g NOT 123 ± 2 g, 70%–85% NOT 70–85%.
● Symbols
Greek letters must be inserted using the correct Greek symbol (using Times, Helvetica or Symbol font), NOT written in full, i.e., alpha: α; beta: β, ß, (available in Times and Helvetica); and gamma: γ, etc.
● Equations
For equations, if preparing your manuscript in Word, use the Microsoft Equation Editor or the MathType add-on. Ensure that equations are editable and not inserted as images.
● Statistical Analysis
Appropriate statistical treatment of the data is essential. When statistical analysis is performed, the name of the statistical test used, the number for each analysis, the comparisons of interest, the alpha level and the actual p-value for each test should be provided.
- When the P value is less than 0.001, report as P < 0.001.
- When the P value is greater than 0.99, report as P > 0.99.
- Special care should be taken when reporting P values of P = 0 or P = 1, as these values are rare and may require additional clarification.
● Scientific Naming and Formatting Rules
Linnean scientific names should be in italics, while higher than generic taxa should not. The generic name of drugs, as well as all other common names, should be written in lower case. Gene designations should be in lower case and in italics, while protein designations should be in regular capital letters. All the p in p value, whether in the text or on the figure, should be lowercase and italic.
For more details and examples, please refer to the template.
Back Matter
● Declarations
Please note that the 6 pieces of information (Acknowledgement, Funding Statement, Author Contributions, Availability of Data and Materials, Ethics Approval, Conflicts of Interest) need to be truthfully provided at the end of the article.
Acknowledgement
This section is intended for acknowledging any support not covered under the Author Contributions or Funding Statement sections. This may include administrative and technical assistance, as well as in-kind contributions such as materials or equipment provided for the research. Please be aware that the specific funding grant number should only appear in the Funding Statement. If there are no acknowledgments to be made, please use “Not applicable”.
Funding Statement
Authors should describe sources of funding that have supported the work, including specific grant numbers, initials of authors who received the grant, and the URLs to sponsors’ websites: “This research was funded by Name of Funder, grant number xxx” or “The APC was funded by xxx”. If there is no funding support, please write “The author(s) received no specific funding for this study”.
Author Contributions
The Author Contributions statement is mandatory for research articles, except for papers with a single author. It should represent all the authors and is to be included upon submission. All listed authors must have substantially contributed to the manuscript and have approved the final submitted version, which should include a description of each author’s specific work and contribution. We suggest the following format for the contribution statement:
“The authors confirm contribution to the paper as follows: Conceptualization, First-name Lastname1 and First-name Lastname2; methodology, First-name Lastname1; software, First-name Lastname1; validation, First-name Lastname1, First-name Lastname2 and First-name Lastname3; formal analysis, First-name Lastname1; investigation, First-name Lastname1; resources, First-name Lastname1; data curation, First-name Lastname1; writing—original draft preparation, First-name Lastname1; writing—review and editing, First-name Lastname1; visualization, First-name Lastname1; supervision, First-name Lastname1; project administration, First-name Lastname1; funding acquisition, First-name Lastname1. All authors reviewed the results and approved the final version of the manuscript”.
Please turn to the CRediT role descriptors—CRediT for the term explanation.
Availability of Data and Materials
This statement should make clear how readers can access the data used in the study and explain why any unavailable data cannot be released. The following five statements are offered for reference:
- Data openly available in a public repository.
“The data that support the findings of this study are openly available in [repository name] at [URL].” - Data available within the article or its Supplementary Materials.
“The authors confirm that the data supporting the findings of this study are available within the article [and/or] its Supplementary Materials.” - Data available on request from the authors.
“The data that support the findings of this study are available from the Corresponding Author, [author initials], upon reasonable request.” - Data not available due to [ethical/legal/commercial] restrictions.
“Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.” - “Not applicable.” (This article does not involve data availability, and this section is not applicable).
Ethics Approval
Guidelines for ethical approval statements may differ based on the journal, a standard ethical approval statement will usually include:
- Whether or not the study included human or animal subjects. In all cases, the ethical approval status of the work should be stated in the ethical approval statement.
- The committee which approved the study.
- The compliance documents. What policies, declarations, acts, etc.
- Persistent identifier: reference or approval number. Include the registration ID/reference number if applicable.
- “Not applicable.” for studies not involving humans or animals.
Conflicts of Interest
Declare conflicts of interest or state: “The author(s) declare(s) no conflicts of interest to report regarding the present study”.
● Supplementary Materials
In addition to the data, computer code, and research materials transparency guidelines, PLANT SCIENCE REVIEW (PSR) encourages authors to provide supplementary materials that complement their main articles and enhance the readers’ understanding of the research. Supplementary materials may include additional data, figures, tables, multimedia content, or relevant information.
- Supplementary Materials Submission: Authors should submit supplementary materials along with their main article during the manuscript submission process. These materials should be in a separate section and clearly labeled as “Supplementary Materials.”.
- Content Relevance: All supplementary materials should be directly relevant to the main research article and provide valuable additional insights or data that support or expand upon the article’s findings. Supplementary materials should not duplicate information already presented in the main text.
- File Formats: Supplementary materials can be submitted in various formats, such as Word, PDF, Excel, CSV, images (JPEG or PNG), audio (MP3), video (MP4), or any other appropriate format for the content type.
- Supplementary Data: Authors can provide raw data or additional data that support the article’s findings but are not included in the main text due to space constraints. Data should be well-organized, properly labeled, and accompanied by clear explanations of the data’s context and significance.
- Supplementary Figures and Tables: Authors may include supplementary figures or tables to complement the main article, which should be clear, readable (with a minimum resolution of 300 dpi), and accompanied by accurate legends. These materials must be referenced in the main text using the prefix “S” (e.g., Fig. S1, Eq. (S2), Table S1) and should be submitted without tracked changes, highlights, comments, or line numbers.
● Appendices
The appendix is an optional section that can contain details and data supplemental to the main text. Authors that need to include an Appendix section should place it before the References section. Multiple appendices should all have headings in the style used for above. They should be ordered as such: A, B, and C, etc.
Appendix sections must be referenced in the main text. Within the appendices, figures, tables, and other elements should be labeled starting with “A”—e.g., Figure A1, Figure A2, etc.
● References
Plant Science Review (PSR) recommends editors and authors to utilize professional reference management tools such as EndNote for academic writing and literature formatting. EndNote is a reference management software from Clarivate Analytics. It is designed to manage bibliographies and references and available for Windows and MacOS. For authors, EndNote offers a convenient and efficient way to format their references according to a particular journal’s guidelines. For journals and publishers, EndNote can save editorial time, and also shortens production time potentially.
All references should be cited in the main text sequentially (including citations in tables and legends) and listed individually at the end of the manuscript. We recommend preparing the references with a bibliography software package, such as EndNote, Mendeley or Zotero, to avoid typing mistakes and duplicated references. Include the digital object identifier (DOI) for all references where available.
For citations of references, please use square brackets and consecutive numbers, e.g., [1], [2,3], [4–6]. For embedded citations in the text with pagination, use both parentheses and brackets to indicate the reference number and page numbers; for example [5] (p. 10), or [6] (p. 101–105). When a cited reference is the subject of a sentence, use the author’s last name (e.g., Rhee [1]) or “Reference/Ref.” (e.g., Reference [1]). For multiple authors, use the first author followed by et al. (e.g., Al-Khshali et al. [2] or Refs. [4–6]). It is not recommended to cite more than 5 consecutive references. Please include the first 6 authors’ names before using “et al.” in the references. Citations and references in the Supplementary Materials are permitted if they also appear in the reference list here.
The following are examples of the reference style (Vancouver style), which should be strictly adhered to (More details of style can refer to: Citing Medicine—NCBI Bookshelf):
● Journals
1. Author AA, Author BB. Title of article. Abbreviated Journal Name. Year;volume(issue):pagination.
2. Author AA Jr, Author BB 2nd, Author CC, Author DD, Author EE, Author FF, et al. Title of article. Abbreviated Journal Name. Year;volume(issue):pagination.
● Books
3. Author AA, Author BB. Title of the book. Publisher Location: Publisher; Year. Pagination (Optional).
4. Editor AA, Editor BB, editors. Title of the book. Publisher Location: Publisher; Year. Pagination (Optional).
5. Author AA, Author BB. Title of the book. xth ed. (if not first) Vol. x (if any). Publisher Location: Publisher; Year. Pagination (Optional).
6. Author AA, Author BB. Title of the book. xth ed. Translator AA, translator. Publisher Location: Publisher; Year. Pagination (Optional).
7. Author AA, Author BB. Title of the book. Publisher Location: Publisher; Year. Section/Table/Charts/… x; Pagination (Required).
8. Author AA, Author BB. Title of the chapter. In: Editor AA, Editor BB, editors. Title of the book. xth ed. (if not first) Publisher Location: Publisher; Year. Pagination (Required).
9. Author AA, Author BB. English Title of the book. Publisher Location: Publisher; Year. Pagination (In Original Language).
● Conferences
10. Editor AA, Editor BB, editors. Book title (Optional). Conference Title: (Proceedings of the) xth Name of Conference; Date of Conference; Location of the Conference (Optional). Publisher Location: Publisher; Year of publication. Pagination (Optional).
11. Author AA, Author BB. Title of the paper. In: Editor AA, Editor BB, editors. Book title (Optional). Conference Title: (Proceedings of the) xth Name of Conference; Date of Conference; Location of the Conference (Optional). Publisher Location: Publisher; Year of publication. Pagination (Required).
12. Author AA, Author BB. Title of the paper/Poster session. Paper/Poster session presented at: Name of the conference; Date of Conference; Location of the Conference (Optional).
● Dissertations and Theses
13. Author AA. Title of dissertation [dissertation/master’s thesis]. Location: Institution Name; Year of publication. Pagination (Optional).
● Web Sites
14. Author AA/Organization (Optional). Title of electronic publication/webpage [Internet/Video/…]. Location: Publisher (Optional); Date of publication (Optional) [cited 2024 Jan 1]. Available from: http://URL.
● Patents
15. Inventor AA, Inventor BB, inventors; Assignee AA, assignee. Title of the patent. Country of patent Patent number. Issue date/grant date.
Research Data and Supplementary Materials
In addition to the data, computer code, and research materials transparency guidelines, Plant Science Review (PSR) encourages authors to provide supplementary materials that complement their main articles and enhance the readers’ understanding of the research. These supplementary materials may include additional data, figures, tables, multimedia content, or relevant information.
- Supplementary Materials Submission: Authors should submit supplementary materials along with their main article during the manuscript submission process. These materials should be in a separate section and clearly labeled as “Supplementary Materials.”
- Content Relevance: All supplementary materials should be directly relevant to the main research article and provide valuable additional insights or data that support or expand upon the article’s findings. Supplementary materials should not duplicate information already presented in the main text.
- File Formats: Supplementary materials can be submitted in various formats, such as Word, PDF, Excel, CSV, images (JPEG or PNG), audio (MP3), video (MP4), or any other appropriate format for the content type.
- Supplementary Data: Authors can provide raw data or additional data that support the article’s findings but are not included in the main text due to space constraints. Data should be well-organized, properly labeled, and accompanied by clear explanations of the data’s context and significance.]
- Supplementary Figures and Tables: Authors may include extra figures or tables that complement those in the main article. These should be numbered separately (e.g., Supplementary Figure S1, Supplementary Table S1) and referred to in the main text.
Chemical Compounds
Chemical and Chemical Nomenclature and Abbreviations
Authors should provide the exact structure of the chemical compound, and if there are appeared as new chemical compounds, authors should submit the small-molecule crystallographic data to the Cambridge Structural Database (CSD) and deposit relevant information to PubChem. The final version of the manuscript should contain the accession codes. When possible, authors should use systematic nomenclature to identify chemical compounds, and biomolecules using IUPAC is preferred. Standard chemical and chemical abbreviations should be used.
Combinatorial Compound Libraries
The authors should include standard characterization data for a diverse panel of library components when describing the preparation of combinatorial libraries in the manuscript.
Chemical Structures for Organic and Organometallic Compounds
Chemical structures for organic and organometallic compounds should be established through spectroscopic analysis. The authors should provide standard peak listings for both 1H NMR and proton-decoupled 13C NMR for all new compounds. Other NMR data, when appropriate, such as 31P NMR, 19F NMR, etc. should be reported. For the identification of functional groups, both UV and IR spectral data should be reported when appropriate. For crystalline materials, melting-point ranges should be included. For the analysis of chiral compounds, specific rotations should be reported. For known compounds, authors should provide detailed references.
Spectral Data
Detailed spectral data for new compounds should be provided in the Materials and methods section. The authors should explain how specific, unambiguous NMR assignments were made in the Materials and methods section.
Crystallographic Data for Small Molecules
For crystallographic data for small molecules, authors should provide a standard crystallographic information file (CIF) and a structural figure with probability ellipsoids. The authors should check the CIF using the International Union of Crystallography (IUCr) checkCIF. For the structure, the structure factors must be included either in the main CIF or in a separate CIF. Crystallographic data for small molecules should be submitted to the Cambridge Crystallographic Data Centre (CCDC), and the accession number must be referenced in the manuscript.
Biomolecular Materials
Manuscripts reporting new biomolecular structures should contain a table summarizing structural and refinement statistics. If suitable, high-field NMR or X-ray crystallography may also be used. For new biopolymeric materials (e.g., oligosaccharides, peptides, nucleic acids, etc.), if it is not possible for structural analysis by NMR spectroscopic methods. Authors must provide evidence of the identity based on sequence (when appropriate) and mass spectral characterization.
Biological Constructs
Authors should provide sequencing or functional data that validates the identity of their biological constructs (plasmids, fusion proteins, site-directed mutants) upon request.
Polymers
For new materials, as well as 1H NMR and 13C NMR, the mass spectral analysis should be used to support the identification of molecular weight. Ideally, high-resolution mass spectral (HRMS) data are preferred.
Nanomaterials
The authors must provide a detailed characterization of both individual objects and bulk composition.
Data Sharing and Deposition
Plant Science Review takes the responsibility to enforce a rigorous peer-review together with strict ethical policies and standards to ensure adding the highest quality scientific works to the field of scholarly publication. PLANT SCIENCE REVIEW (PSR) takes such publishing ethics issues very seriously on every level. Our staff are trained to identify and report any irregularities. Our editors follow Guidelines of the Committee on Publication Ethics (COPE), and proceed with a zero tolerance policy for ethical violations, including plagiarism, data falsification and authorship misconduct. To confirm the originality of all submitted manuscripts, we use iThenticate for similarity checks against prior publications.
